171 | Add to Reading ListSource URL: www.research.uky.eduLanguage: English - Date: 2015-12-22 08:52:16
|
|---|
172 | Add to Reading ListSource URL: www.neovictorianstudies.comLanguage: English - Date: 2010-11-19 10:08:35
|
|---|
173 | Add to Reading ListSource URL: www.firstclinical.comLanguage: English - Date: 2014-09-14 03:12:34
|
|---|
174 | Add to Reading ListSource URL: www.stc2016.orgLanguage: English |
|---|
175 | Add to Reading ListSource URL: www.kofam.chLanguage: English - Date: 2016-05-30 02:33:37
|
|---|
176 | Add to Reading ListSource URL: magiworld.orgLanguage: English - Date: 2015-12-14 16:21:29
|
|---|
177 | Add to Reading ListSource URL: si.deis.unical.itLanguage: English |
|---|
178 | Add to Reading ListSource URL: people.westminstercollege.eduLanguage: English - Date: 2006-05-24 14:36:02
|
|---|
179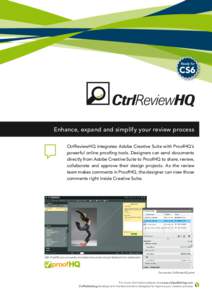 | Add to Reading ListSource URL: www.proofhq.comLanguage: English - Date: 2015-09-24 14:08:04
|
|---|
180 | Add to Reading ListSource URL: humansubjects.stanford.eduLanguage: English - Date: 2016-04-12 01:21:51
|
|---|